Feeding the Turbo Rotary: Horsepower, Airflow, Fuels
#51
re. exchange above...
You have to consider where the vaporization happen and what it causes as a result.
1. Injected as a liquid directly into or very close to the combustion chamber and all vaporizing inside it: all the calculations above are correct, water cools better, then alcohol, then gasoline.
2. Injected before proximity to the combustion chamber. Depending on location, droplet atomization, temperature, pressure, and initial air humidity, some of the liquid vaporizes before entering the combustion chamber and cools the intake air down (and - hopefully - the air being compressed inside the compressor as well if injected pre-compressor...), which can cause the IAT to decrease, depending on how much liquid has actually changed phase before entering the combustion chamber.
3. Any liquid that vaporized already before entering the combustion chamber has already absorbed the BTU associated to its latent heat of vaporization and just became one of the gas in the air mixture. The associated BTU amount is no longer available to further absorb heat and cooling.
4. Often forgotten, when water or alcohol vaporize before entering the combustion chamber, it causes dilution of the O2 available for combustion, which - everything else equal - reduces the energy released during combustion. In general, 2. and 4. tend to offset each other. As liquid vaporizes it causes cooldown, which increases air density and therefore more O2 density.
An excellent - although simplified - calculator which can help finding the boundaries is here.
https://www.rx7club.com/showthread.p...=1#post9767217
Make sure you read the limitations of the model. In particular as it concerns 2. and 4. above.
- Sandro
You have to consider where the vaporization happen and what it causes as a result.
1. Injected as a liquid directly into or very close to the combustion chamber and all vaporizing inside it: all the calculations above are correct, water cools better, then alcohol, then gasoline.
2. Injected before proximity to the combustion chamber. Depending on location, droplet atomization, temperature, pressure, and initial air humidity, some of the liquid vaporizes before entering the combustion chamber and cools the intake air down (and - hopefully - the air being compressed inside the compressor as well if injected pre-compressor...), which can cause the IAT to decrease, depending on how much liquid has actually changed phase before entering the combustion chamber.
3. Any liquid that vaporized already before entering the combustion chamber has already absorbed the BTU associated to its latent heat of vaporization and just became one of the gas in the air mixture. The associated BTU amount is no longer available to further absorb heat and cooling.
4. Often forgotten, when water or alcohol vaporize before entering the combustion chamber, it causes dilution of the O2 available for combustion, which - everything else equal - reduces the energy released during combustion. In general, 2. and 4. tend to offset each other. As liquid vaporizes it causes cooldown, which increases air density and therefore more O2 density.
An excellent - although simplified - calculator which can help finding the boundaries is here.
https://www.rx7club.com/showthread.p...=1#post9767217
Make sure you read the limitations of the model. In particular as it concerns 2. and 4. above.
- Sandro
#52
1. My main purpose at this time is to see if it is possible to achieve enough evaporative cooling inside the compressor to significantly move the compression toward isothermal condition.
2. In order to achieve that I know for sure I need very good atomization. Based on what I have learned but more on what I have not-learned (lack of data), I am not convinced that the air/water nozzles operated under our conditions can achieve a degree of atomization better the than the Bete impingement nozzles. It appears you would need at least 400-1000 air/water ratio to get to "fog" type droplets. Spraying System indicated I could expect no better than 80-90 micron. I would love to see a bench test of the SUE18A nozzle simulating our conditions.
3. Using a pump and the Bete PJ nozzles make it easier to assemble, change, inspect, etc. in particular with my TT sequential set-up and for my test objectives
4. I have no reliability or low-complexity goals. My car is a dedicated autoxer and I am not thinking of any retuning for now.
Having said that, the Rice Racing, the "Dudemaaanownsanrx7" and others based on the same concepts utilizing the atomizing air/water nozzle are proved to be working well overall and clearly deserve positive considerations.
- Sandro
#53
On flats
iTrader: (29)
Join Date: Oct 2004
Location: Albuquerque
Posts: 1,379
Likes: 0
Received 0 Likes
on
0 Posts
You would literally need to be pumping in volumes of water comparable to the CFMs of air your motor is using before what you are saying becomes true.
#54
re. exchange above...
An excellent - although simplified - calculator which can help finding the boundaries is here.
https://www.rx7club.com/showthread.p...=1#post9767217
Make sure you read the limitations of the model. In particular as it concerns 2. and 4. above.
- Sandro
An excellent - although simplified - calculator which can help finding the boundaries is here.
https://www.rx7club.com/showthread.p...=1#post9767217
Make sure you read the limitations of the model. In particular as it concerns 2. and 4. above.
- Sandro
http://not2fast.com/thermo/water_inj...opt_mass.shtml
and this is in re. to the thermodynamic equations used in the model, including the calculation (through the law of partial pressure) of the O2 displaced, even though it look like someone seems having some strong different opinion about it...
http://not2fast.com/thermo/water_inj...njection.shtml
- Sandro
#55
This isn't true, and especially not speaking in practical terms. Familiarize yourself with the law of partial pressures.
You would literally need to be pumping in volumes of water comparable to the CFMs of air your motor is using before what you are saying becomes true.
You would literally need to be pumping in volumes of water comparable to the CFMs of air your motor is using before what you are saying becomes true.
The order of magnitude of the ratio between specific volumes of vapor and liquid is 1,000.
So, just 1/1,000 of liquid (in volume) makes a match.
Air contains 20% of O2. Assuming 100% vaporization of just an additional 1/1,000 of liquid in volume, you would then dilute the O2 in 1/2
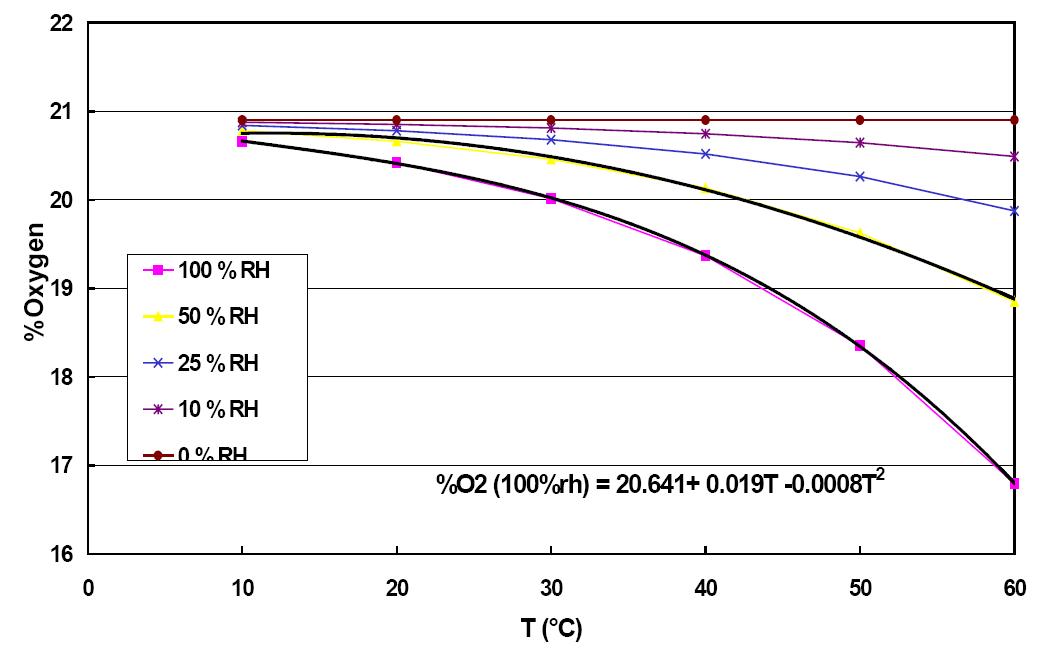
The chart below shows directly O2 dilution at different humidity. Most likely, this was charted at barometric pressure. As temperature increases, you can have more water vapor in it. Note how you can extrapolate that at 100 deg C (vapor partial pressure equals boiling point) with 100% humidity O2 concentration does approach the 10% simplistically calculated above.
Again, you can refer to the links I provided in my earlier post and see the results of the calculations and the thermodynamics laws the model is based on.
#56
On flats
iTrader: (29)
Join Date: Oct 2004
Location: Albuquerque
Posts: 1,379
Likes: 0
Received 0 Likes
on
0 Posts
The assumption that air is displaced is what is flawed.
The use of specific volumes, while intuitively might seem to add up, is physically incorrect in so far as implicit in it is the assumption that the mass is fixed. Each component of the mixture has a specific volume, absolutely. The mass, however, is NOT fixed, the volume is. Consequently, the specific volume of the mixture simply increases. There are still just as many O2 molecules per volume as there were pre-injectant, there are just ALSO a certain number of H2O molecules. Thusly the specific volume of the mixture is increased with no dilution.
I'll do my best to pose a scenario that lends itself to easy physical interpretation. Let's consider a regular gasoline only engine. Lets ignore everything, intake-wise, before the valve/port. At a given RPM/load, a certain amount of air is entering the engine. Fuel is injected. According to your conjecture, some of the air is then removed by virtue of the fuel having been injected. Where does it go?
Another question worth considering:
Why don't any cars have hygrometers as regular instrumentation for the ECU?
The use of specific volumes, while intuitively might seem to add up, is physically incorrect in so far as implicit in it is the assumption that the mass is fixed. Each component of the mixture has a specific volume, absolutely. The mass, however, is NOT fixed, the volume is. Consequently, the specific volume of the mixture simply increases. There are still just as many O2 molecules per volume as there were pre-injectant, there are just ALSO a certain number of H2O molecules. Thusly the specific volume of the mixture is increased with no dilution.
I'll do my best to pose a scenario that lends itself to easy physical interpretation. Let's consider a regular gasoline only engine. Lets ignore everything, intake-wise, before the valve/port. At a given RPM/load, a certain amount of air is entering the engine. Fuel is injected. According to your conjecture, some of the air is then removed by virtue of the fuel having been injected. Where does it go?
Another question worth considering:
Why don't any cars have hygrometers as regular instrumentation for the ECU?
Last edited by calculon; 01-28-10 at 12:21 PM.
#57
The assumption that air is displaced is what is flawed.
The use of specific volumes, while intuitively might seem to add up, is physically incorrect in so far as implicit in it is the assumption that the mass is fixed. Each component of the mixture has a specific volume, absolutely. The mass, however, is NOT fixed, the volume is. Consequently, the specific volume of the mixture simply increases. There are still just as many O2 molecules per volume as there were pre-injectant, there are just ALSO a certain number of H2O molecules. Thusly the specific volume of the mixture is increased with no dilution.
I'll do my best to pose a scenario that lends itself to easy physical interpretation. Let's consider a regular gasoline only engine. Lets ignore everything, intake-wise, before the valve/port. At a given RPM/load, a certain amount of air is entering the engine. Fuel is injected. According to your conjecture, some of the air is then removed by virtue of the fuel having been injected. Where does it go?
The use of specific volumes, while intuitively might seem to add up, is physically incorrect in so far as implicit in it is the assumption that the mass is fixed. Each component of the mixture has a specific volume, absolutely. The mass, however, is NOT fixed, the volume is. Consequently, the specific volume of the mixture simply increases. There are still just as many O2 molecules per volume as there were pre-injectant, there are just ALSO a certain number of H2O molecules. Thusly the specific volume of the mixture is increased with no dilution.
I'll do my best to pose a scenario that lends itself to easy physical interpretation. Let's consider a regular gasoline only engine. Lets ignore everything, intake-wise, before the valve/port. At a given RPM/load, a certain amount of air is entering the engine. Fuel is injected. According to your conjecture, some of the air is then removed by virtue of the fuel having been injected. Where does it go?
But in Case 4. there is no "fixed volume", closed chamber as water becomes vapor. The best way I can offer you to figure this out, is to think in terms of molecular matter been injected e.g. pre-com (i.e. mass) and then think at the relative specific volume of each of the constituents before and after vaporization. And yes, the mass flow rates are fixed (constant amount of air flow entering the intake and same amount of water flow). But now, as liquid vaporizes, the relative specific volumes of its constituents changes. And since now the same mass of water occupies more space, the relative amount of O2 entering the combustion chamber is lower. I hope this helps.
- Sandro
#58
The assumption that air is displaced is what is flawed.
The use of specific volumes, while intuitively might seem to add up, is physically incorrect in so far as implicit in it is the assumption that the mass is fixed. Each component of the mixture has a specific volume, absolutely. The mass, however, is NOT fixed, the volume is. Consequently, the specific volume of the mixture simply increases. There are still just as many O2 molecules per volume as there were pre-injectant, there are just ALSO a certain number of H2O molecules. Thusly the specific volume of the mixture is increased with no dilution.
I'll do my best to pose a scenario that lends itself to easy physical interpretation. Let's consider a regular gasoline only engine. Lets ignore everything, intake-wise, before the valve/port. At a given RPM/load, a certain amount of air is entering the engine. Fuel is injected. According to your conjecture, some of the air is then removed by virtue of the fuel having been injected. Where does it go?
The use of specific volumes, while intuitively might seem to add up, is physically incorrect in so far as implicit in it is the assumption that the mass is fixed. Each component of the mixture has a specific volume, absolutely. The mass, however, is NOT fixed, the volume is. Consequently, the specific volume of the mixture simply increases. There are still just as many O2 molecules per volume as there were pre-injectant, there are just ALSO a certain number of H2O molecules. Thusly the specific volume of the mixture is increased with no dilution.
I'll do my best to pose a scenario that lends itself to easy physical interpretation. Let's consider a regular gasoline only engine. Lets ignore everything, intake-wise, before the valve/port. At a given RPM/load, a certain amount of air is entering the engine. Fuel is injected. According to your conjecture, some of the air is then removed by virtue of the fuel having been injected. Where does it go?
But in Case 4. there is no "fixed volume", closed chamber as water becomes vapor. The best way I can offer you to figure this out, is to think in terms of molecular matter been injected e.g. pre-com (i.e. mass) and then think at the relative specific volume of each of the constituents before and after vaporization. And yes, the mass flow rates are fixed (constant amount of air flow entering the intake and same amount of water flow). But now, as liquid vaporizes, the relative specific volumes of its constituents changes. And since now the same mass of water occupies more space, the relative amount of O2 entering the combustion chamber is lower. I hope this helps.
- Sandro
With reference to my initial post #51, Case 1. --- falls under the very same scenario you just described and there is no O2 dilution to consider there, obviously.
But in --- Case 2. there is no "fixed volume", closed chamber as water becomes vapor. The best way I can offer you to figure this out, is to think in terms of molecular matter been injected e.g. pre-com. (i.e. mass) and then think at the relative specific volume of each of the constituents before and after vaporization. And yes, the mass flow rates are fixed (constant amount of air flow entering the intake and same amount of water flow). But now, as liquid vaporizes, the relative specific volumes of its constituents changes. And since now the same mass of water occupies more space, the relative amount of O2 entering the combustion chamber is lower. I hope this helps.
- Sandro
#59
On flats
iTrader: (29)
Join Date: Oct 2004
Location: Albuquerque
Posts: 1,379
Likes: 0
Received 0 Likes
on
0 Posts
A molecule of H2O occupies the EXACT same amount of space whether it is as a vapor or as a liquid. This can only change if it enters into a crystal lattice as it does to become ice. (Even then, a single molecule still only occupies a given amount of space, but the lattice itself has a geometry that makes the average size increase, in the case of water)
With regards to there being no fixed volume, that's just not true. The intake tract has a fixed volume which the air being fed by the turbo and the water being injected occupy. What you are suggesting would require air to be forced OUT of the intake tract as water/fuel is injected. This doesn't happen.
The questions I asked weren't rhetorical. In maintaining your position, you should be able to answer them.
With regards to there being no fixed volume, that's just not true. The intake tract has a fixed volume which the air being fed by the turbo and the water being injected occupy. What you are suggesting would require air to be forced OUT of the intake tract as water/fuel is injected. This doesn't happen.
The questions I asked weren't rhetorical. In maintaining your position, you should be able to answer them.
Let's consider a regular gasoline only engine. Lets ignore everything, intake-wise, before the valve/port. At a given RPM/load, a certain amount of air is entering the engine. Fuel is injected. According to your conjecture, some of the air is then removed by virtue of the fuel having been injected. Where does it go?
Why don't any cars have hygrometers as regular instrumentation for the ECU?
#60
REST IN PEACE DAVE!!!!!!
iTrader: (7)
Join Date: Sep 2003
Location: las vegas.nevada.
Posts: 1,673
Likes: 0
Received 0 Likes
on
0 Posts
I was tossing this around in my head also. pre- or post turbo. i keep reading about the pre and i think im going that route also.
what cc nozzel should i get running 15-18psi on pump gas with 550 primaries and 1680 secondaries and a to4r
also if i were to get a cooling mist system would i still need an atomizer?
what cc nozzel should i get running 15-18psi on pump gas with 550 primaries and 1680 secondaries and a to4r
also if i were to get a cooling mist system would i still need an atomizer?
#61
Rotary Enthusiast
OK! air is N2O, that says 33% oxygen, and water is H2O, that says 33% oxygen, question how do these two relate to each in gasous form?? in the process of combustion and (most important) EXPANSION>
tests hve shown, water expanse 1700 times its original volume at 212*F,it is visible as steam, but at around 1000*F it becomes superheated and goes non-visible.
does it expand more? sometimes we lose sight of exactly what we want to do,
in an ICE, its all about CONTROLLED expansion and make torque at output shaft.
tests hve shown, water expanse 1700 times its original volume at 212*F,it is visible as steam, but at around 1000*F it becomes superheated and goes non-visible.
does it expand more? sometimes we lose sight of exactly what we want to do,
in an ICE, its all about CONTROLLED expansion and make torque at output shaft.
#62
A molecule of H2O occupies the EXACT same amount of space whether it is as a vapor or as a liquid. This can only change if it enters into a crystal lattice as it does to become ice. (Even then, a single molecule still only occupies a given amount of space, but the lattice itself has a geometry that makes the average size increase, in the case of water)
With regards to there being no fixed volume, that's just not true. The intake tract has a fixed volume which the air being fed by the turbo and the water being injected occupy. What you are suggesting would require air to be forced OUT of the intake tract as water/fuel is injected. This doesn't happen.
The questions I asked weren't rhetorical. In maintaining your position, you should be able to answer them.
With regards to there being no fixed volume, that's just not true. The intake tract has a fixed volume which the air being fed by the turbo and the water being injected occupy. What you are suggesting would require air to be forced OUT of the intake tract as water/fuel is injected. This doesn't happen.
The questions I asked weren't rhetorical. In maintaining your position, you should be able to answer them.
http://www.efunda.com/materials/wate...mtable_sat.cfm
2. Your example of fuel being injected, etc. where does the air goes? is equivalent to my Case no. 1 which i already discussed earlier while describing the effect of phase change in water. The answer is therefore the same. Since vaporization happens exclusively inside the combustion chamber, there is no change in the number of O2 molecules obviously. All the O2 molecules were already "trapped" before the vaporization happened.
3. For the case no. 2 (i.e. evaporation inside the intake), I never said that the volume of the piping is not constant. The distinction I made is that it is not an enclosed system like the combustion chamber and that the mass balance - not the volume balance - must be mantained. There is an intake sucking air at atmospheric pressure, a compressor, other piping and restrictions that cause pressure losses and an intake manifold which we can consider at fixed pressure, as determined by the boost control system. The combination of these components determines how much air (in volume like cfm) enters the combustion chamber irrespective from the relative composition of gases in the air or its density. However, the amount of O2 entering the CC is very much dependent on the air composition and its density.
Example: if we pump in hotter air (ex. a less effective I/C), its density at the intake manifold will be lower (pv=RT; density is 1/v), and less molecules of O2 will enter the CC. That is why colder is better. Now, I can perhaps see where your doubts lie. You may be thinking: but if I have 500 cfm of hot air entering the CC, shouldn't I also have 500 cfm of fresh air entering the intake? Well...the answer is no. The mass amount will be the same (what comes into the intake has to go into the CC), and so will be the O2 amount. But the volumetric flow of fresh air will be 500 cfm time the ratio of the densities (X cfm Fresh air x density fresh air = 500 cfm x density intake air, this is the mass balance that must be met). By the same token we can also conclude that the amount of O2 molecules entering the air intake is actually controlled by the density (i.e. the combination of pressure and temperature) at the intake manifold.
Now, let's jump into a parallel case where we inject liquid water this time, then have a H2O change of phase in between the air intake and the intake manifold (enough thermal energy absorbed by the liquid water to cause it to vaporize). This time we are actually changing the gas composition of the air. We have a new gas, water vapor, in it. The relative composition of gases has now changed, in particular the % of O2 has decreased. For reference, look again at the chart in my previous post #55. So, you may have a similar question. How is this possible? Where did all the O2 I sucked at the air intake go? Once again, the answer is that the mass balances and the conditions at the intake manifold dictate the amount of fresh air sucked from the atmosphere. Believe it or not, because now we have this new gas "created" inside, we need less fresh air coming in. But less fresh air coming in also means less O2. Note that all the mass balance are maintained. The same mass amount of water we injected enters the CC although in the form of a gas. The same mass amount of fresh air enters the CC as well. Too bad that this time less fresh air (and therefore less O2) is needed to keep the balance in check.
Calculon, it has been an interesting discussion but I propose to stop it here now, as we have been monopolizing this thread.
This is not a beauty contest nor I am on a mission to convince you otherwise. If you think I am wrong, so be it.
My purpose was only to contribute to the discussion by sharing my thought and trying to provide relatively simple explanations to mechanisms that are objectively quite complex. I invite you once again to read the links I referred to in my post #54, which I found excellent.
On the other hand, please feel free to PM me directly you wish to continue this discussion off-line.
Also, I wanted to apologize to Horward. It was not my intention to highjack your thread.
- Sandro
#63
BDC Motorsports
That was when I had the two M10 nozzles located at the tail end of the hot-air pipe just shy of the throttle body inlet, perhaps about 6". Once I moved the nozzles well up-stream, with the same parameters running, the air temps at the same boost dropped to a few degrees below ambient and there was no more knock. It ran just fine. It never made that "clak clak clak" rattle again.
B
#66
On flats
iTrader: (29)
Join Date: Oct 2004
Location: Albuquerque
Posts: 1,379
Likes: 0
Received 0 Likes
on
0 Posts
With respect to monopolizing this thread, I disagree that we've been at all inappropriate or inconsiderate to explore this topic. After all, what we are discussing is tantamount to the thread's subject. I, like you I believe, am only interested in having the objective truth spelled out for everyone's benefit. It is not my perception that this is, or my wish to make it a, as you put it "beauty contest." I have, in fact, read the articles that you asked me to. I have also pointed out the mistake in their assumption set that led to the erroneous conclusions.
I didn't say that they did. Please reread what I did say.
The volume of the piping IS constant though, isn't it? Please explain how the intercooler piping, end tanks, core, intake plenum, and runners change volume in any substantive way.
This is not true. Adding water (or ANYTHING more dense than air, for that matter) increases the mass of the charge. Being that the volume IS fixed, the pressure and temperature of the mixture must change. As this happens, components of the mixture may undergo a phase change as dictated by their phase diagrams. This forced thermodynamic balance is the fundamental motive force that leads to the associated temperature drops.
In order to resolve this, we must be able to answer each others' questions while sticking to our respective positions. If our position is valid, there is no reason why these questions should be unable to be answered. That said, I'd like to pose some more for you.
According to the Excel plot you posted, at ~%100 relative humidity, the amount of oxygen in the air varies from ~20.5% to ~16.5% with a change from ~50'F to ~140'F. Air EASILY sees this type of heating from compression in a turbocharger. In reality, it sees more, making the illustration I'm about to make grossly conservative.
Lets remember that 100% relative humidity is a common atmospheric condition. It happens every time that it is just about to start raining. Surely, this is a condition that we've ALL driven our turbocharged cars in.
Lets also remember that lambda sensors (AFR meters) work by detecting oxygen richness of the charge exiting the engine.
16.5/20.5 = ~80% . . . There is ~80% less oxygen available in the air as a result of this temperature change.
Since our cars aren't equipped with hygrometers, they have no way to know that the relative humidity is ~100%. Consequently they keep on injecting fuel and firing our plugs as they've been told to as functions of RPM and load (and maybe some other fancy trims if we're clever). Can you please explain why our AFRs stay the same and our cars don't bog from this very effectively rich condition?
So again, please simply answer these questions:
1) The volume of the piping IS constant though, isn't it? Please explain how the intercooler piping, end tanks, core, intake plenum, and runners change volume in any substantive way.
2) With regards to my example of driving in the rain, can you please explain why our AFRs stay the same and our cars don't bog from this very effectively rich condition?
Thank you for the exchange. I do honestly believe that it is worthwhile to have these matters resolved so as to limit the continued spread of disinformation. Especially with motors as sensitive as ours.
Cheers.
ryan
Edit:
In having had these types of discussions before, I understand fully that perhaps what is failing me is my ability to articulate. That said, please read the subsection titled "A common misconception" in THIS LINK. I know it's wikipedia, but it's easily as legitimate (in actuality more so because it is peer reviewed) as any other internet source. It describes, hopefully in a way that makes more sense, what it was I was trying to get across by asking the reader to familiarize themselves with the law of partial pressures.
Originally Posted by calculon
A molecule of H2O occupies the EXACT same amount of space whether it is as a vapor or as a liquid.
In order to resolve this, we must be able to answer each others' questions while sticking to our respective positions. If our position is valid, there is no reason why these questions should be unable to be answered. That said, I'd like to pose some more for you.
According to the Excel plot you posted, at ~%100 relative humidity, the amount of oxygen in the air varies from ~20.5% to ~16.5% with a change from ~50'F to ~140'F. Air EASILY sees this type of heating from compression in a turbocharger. In reality, it sees more, making the illustration I'm about to make grossly conservative.
Lets remember that 100% relative humidity is a common atmospheric condition. It happens every time that it is just about to start raining. Surely, this is a condition that we've ALL driven our turbocharged cars in.
Lets also remember that lambda sensors (AFR meters) work by detecting oxygen richness of the charge exiting the engine.
16.5/20.5 = ~80% . . . There is ~80% less oxygen available in the air as a result of this temperature change.
Since our cars aren't equipped with hygrometers, they have no way to know that the relative humidity is ~100%. Consequently they keep on injecting fuel and firing our plugs as they've been told to as functions of RPM and load (and maybe some other fancy trims if we're clever). Can you please explain why our AFRs stay the same and our cars don't bog from this very effectively rich condition?
So again, please simply answer these questions:
1) The volume of the piping IS constant though, isn't it? Please explain how the intercooler piping, end tanks, core, intake plenum, and runners change volume in any substantive way.
2) With regards to my example of driving in the rain, can you please explain why our AFRs stay the same and our cars don't bog from this very effectively rich condition?
Thank you for the exchange. I do honestly believe that it is worthwhile to have these matters resolved so as to limit the continued spread of disinformation. Especially with motors as sensitive as ours.
Cheers.
ryan
Edit:
In having had these types of discussions before, I understand fully that perhaps what is failing me is my ability to articulate. That said, please read the subsection titled "A common misconception" in THIS LINK. I know it's wikipedia, but it's easily as legitimate (in actuality more so because it is peer reviewed) as any other internet source. It describes, hopefully in a way that makes more sense, what it was I was trying to get across by asking the reader to familiarize themselves with the law of partial pressures.
Last edited by calculon; 01-29-10 at 05:20 PM.
#67
Methanol has its drawbacks, but is pale in comparison to its advantages. Like many of us know, methanol as a fuel is corrosive to some metals such as aluminum. Methyl alcohol (methanol) attacks the oxide coating that normally protect the aluminum from corrosion, such as aluminum intercooler piping with AI, or housings, and most rubbers.
Question #1
When methanol is diluted with water (approx 50/50) and then injected through the nozzles, thus atomizing (vapor), is this still corrosive to the aluminum piping/throttle body/housings? Or is it just a little corrosive with little to no problems with longevity of the engine?
Am I worrying too much?
(Sorry, I'm not able to articulate any better than this)
I know that when turbo/supercharging any engine, the longevity decreases due to obvious reasons (engine ware) and that people don't turbocharge their vehicles to support 450hp+ for their longevity as per say to have the sensational feeling of power/"bone chilling fun". But for me I barely have the funding to finish my project and what I worry about this car is just becoming a quick buzz instead of a longer lasting joy ride.
There is also the case for running Ethanol (E85), but due to its corrosive nature towards rubbers/fuel tank linings/fuel lines/etc, I sort of leaning more towards 50/50 "methwater".
What I have read/and understood, ethanol is a versatile solvent miscible with water and with many organic solvents. E85 which typically contains a mixture of up to 85% denatured fuel ethanol and gasoline have been used in rotary's and other forced induction vehicles, but I've heard about O-rings falling apart in their micron filters, hoses failing after a short period of time, fuel tank lining being eaten away and thus getting pushed through the fuel pump etc.
Do the pros outweigh the cons ^?
When putting these two together (E85/premix) in the gas tank, what happens to this mixture? Does it mix evenly?
What happens to the oil film on aluminum housings when using E85/Premix?
Premix allows for an even distribution of oil on the surface of the housings but ethanol acts as a solvent to "loosen" the oil off of the housing, am I interpreting this wrong?
I need help choosing a fuel
These are the current mods:
S5 13B TII with stock ports
Aeromotive A1000 fuel pump
850cc/1200cc injectors
SS/ braided fuel lines
Sump fuel tank with no lining
44mm external waste-gate with 10psi spring
Master Power T61 turbo (70mm) min of 15psi max of 25psi
MSII V3.0 EMS
Stock coils
Front mount intercooler
NGK AFX Wideband controller
electronic boost controller
what should I do in my case? what octane fuel/E85/Methanol
I know I'm asking for a lot of answers but I want your wisdom to help me out so that I may not regret going a certain route. I don't mean to thread jack and thank you for your help.
Question #1
When methanol is diluted with water (approx 50/50) and then injected through the nozzles, thus atomizing (vapor), is this still corrosive to the aluminum piping/throttle body/housings? Or is it just a little corrosive with little to no problems with longevity of the engine?
Am I worrying too much?
(Sorry, I'm not able to articulate any better than this)
I know that when turbo/supercharging any engine, the longevity decreases due to obvious reasons (engine ware) and that people don't turbocharge their vehicles to support 450hp+ for their longevity as per say to have the sensational feeling of power/"bone chilling fun". But for me I barely have the funding to finish my project and what I worry about this car is just becoming a quick buzz instead of a longer lasting joy ride.
There is also the case for running Ethanol (E85), but due to its corrosive nature towards rubbers/fuel tank linings/fuel lines/etc, I sort of leaning more towards 50/50 "methwater".
What I have read/and understood, ethanol is a versatile solvent miscible with water and with many organic solvents. E85 which typically contains a mixture of up to 85% denatured fuel ethanol and gasoline have been used in rotary's and other forced induction vehicles, but I've heard about O-rings falling apart in their micron filters, hoses failing after a short period of time, fuel tank lining being eaten away and thus getting pushed through the fuel pump etc.
Do the pros outweigh the cons ^?
When putting these two together (E85/premix) in the gas tank, what happens to this mixture? Does it mix evenly?
What happens to the oil film on aluminum housings when using E85/Premix?
Premix allows for an even distribution of oil on the surface of the housings but ethanol acts as a solvent to "loosen" the oil off of the housing, am I interpreting this wrong?
I need help choosing a fuel
These are the current mods:
S5 13B TII with stock ports
Aeromotive A1000 fuel pump
850cc/1200cc injectors
SS/ braided fuel lines
Sump fuel tank with no lining
44mm external waste-gate with 10psi spring
Master Power T61 turbo (70mm) min of 15psi max of 25psi
MSII V3.0 EMS
Stock coils
Front mount intercooler
NGK AFX Wideband controller
electronic boost controller
what should I do in my case? what octane fuel/E85/Methanol
I know I'm asking for a lot of answers but I want your wisdom to help me out so that I may not regret going a certain route. I don't mean to thread jack and thank you for your help.
#68
The housings have iron sleeve inserts that are chromed.
For methanol or E85 you would want to use fluorosilicone O-rings.
I don't recall, but is the A1000 E85 compatible?
I hear nothing but good things about E85 from first hand users, but have not used it personally. Looking at your injectors, I think if you go this way you will need more capacity.
For methanol or E85 you would want to use fluorosilicone O-rings.
I don't recall, but is the A1000 E85 compatible?
I hear nothing but good things about E85 from first hand users, but have not used it personally. Looking at your injectors, I think if you go this way you will need more capacity.
#70
The housings have iron sleeve inserts that are chromed.
For methanol or E85 you would want to use fluorosilicone O-rings.
I don't recall, but is the A1000 E85 compatible?
I hear nothing but good things about E85 from first hand users, but have not used it personally. Looking at your injectors, I think if you go this way you will need more capacity.
For methanol or E85 you would want to use fluorosilicone O-rings.
I don't recall, but is the A1000 E85 compatible?
I hear nothing but good things about E85 from first hand users, but have not used it personally. Looking at your injectors, I think if you go this way you will need more capacity.
I already knew the A1000 fuel pump is compatible but my micron filter says it isn't (its the type where you can replace the filter) probably because of the rubber o-rings in the casing.
What does you setup consist of (as in where you place the nozzles, pump, etc)?
if you have pics or if it is in a different thread, please show me some visible clues.
Thank you very much...this thread has really helped me out and has gotten the gerbil in my head spinning.
#71
Maybe try contacting injector dynamics. I've found those guys to be helpful and they have rotary experience. Or search around here, there probably is a forum member that has posted bsfc equations related to the rotary and E85.
#72
I run 93 octane, just whatever place in town has it cheapest. I run a mechanical water injection so there is no pump. The tank is mounted in the engine bay and the nozzle is located in front of the turbo. The air pressure from the turbo pressurizes the tank and is what actually feeds water into the nozzle. Very reliable way of setting up aux injection.
Here is the kit: http://www.wannaspeed.com/index.php?...roducts_id=181
here is my original build up of the kit: https://www.rx7club.com/auxiliary-injection-173/going-make-my-own-wi-kit-807016/
And rdahm has some youtube videos up of his install of the mechanical water injection kit. His youtube name is emerciv
Here is the kit: http://www.wannaspeed.com/index.php?...roducts_id=181
here is my original build up of the kit: https://www.rx7club.com/auxiliary-injection-173/going-make-my-own-wi-kit-807016/
And rdahm has some youtube videos up of his install of the mechanical water injection kit. His youtube name is emerciv
#73
I run 93 octane, just whatever place in town has it cheapest. I run a mechanical water injection so there is no pump. The tank is mounted in the engine bay and the nozzle is located in front of the turbo. The air pressure from the turbo pressurizes the tank and is what actually feeds water into the nozzle. Very reliable way of setting up aux injection.
Here is the kit: http://www.wannaspeed.com/index.php?...roducts_id=181
here is my original build up of the kit: https://www.rx7club.com/showthread.php?t=807016
And rdahm has some youtube videos up of his install of the mechanical water injection kit. His youtube name is emerciv
Here is the kit: http://www.wannaspeed.com/index.php?...roducts_id=181
here is my original build up of the kit: https://www.rx7club.com/showthread.php?t=807016
And rdahm has some youtube videos up of his install of the mechanical water injection kit. His youtube name is emerciv
#74
Arrogant Wankeler
To those arguing of "fixed intake volume" and relative humidity.
Two very simple points
1. It is a dynamic tract, and the compressor is not fixed displacement, if you are running a set pressure, depending on what injectants expand on absorbing heat in the intake you will get a different mass flow rate from the compressor at the same rpm & load (boost) to balance the fixed volume flow rate of the engine V injectant + air.
2. You are all running at 1-3 bar boost pressures, the partial pressure of water at ~ 2.5 atmospheres represents a much lesser portion of the total system pressure as compared to atmospheric conditions.
The humidity in the air increases the intercooling effect thanks to flow across the outside of an intercooler (having a slightely higher thermal capacity) & reduces the heat increase from compression thanks to its thermal properties, giving no significant loss of performance, unless you are at choke flow at the compressor and are thus reducing the equivalent "dry" air mass flow at the compressor, but unless it is very warm ambient it will be marginal anyway.
Two very simple points
1. It is a dynamic tract, and the compressor is not fixed displacement, if you are running a set pressure, depending on what injectants expand on absorbing heat in the intake you will get a different mass flow rate from the compressor at the same rpm & load (boost) to balance the fixed volume flow rate of the engine V injectant + air.
2. You are all running at 1-3 bar boost pressures, the partial pressure of water at ~ 2.5 atmospheres represents a much lesser portion of the total system pressure as compared to atmospheric conditions.
The humidity in the air increases the intercooling effect thanks to flow across the outside of an intercooler (having a slightely higher thermal capacity) & reduces the heat increase from compression thanks to its thermal properties, giving no significant loss of performance, unless you are at choke flow at the compressor and are thus reducing the equivalent "dry" air mass flow at the compressor, but unless it is very warm ambient it will be marginal anyway.

